Developing Immunotherapies
Oncolytic Virus Therapy in Pediatric Brain Tumors
The goal of this project is to test the hypothesis that a pre-existing anti-tumor immune response will inhibit the killing efficiencies of measles and Toca511 oncolytic viruses in pediatric medulloblastoma, to determine if oncolytic virus therapy is aided by immune checkpoint blockade, and to elucidate the interactions between oncolytic virus and the tumor microenvironment. Our approach will be to apply single-cell and bulk 'omics methods to specimens from an ongoing clinical trial and correlate that with analysis in a patient-relevant mouse model.
The Diaz Lab will perform single-cell and bulk 'omics assays on specimens from patients in a clinical trial run by Dr. Mueller, and on murine tissues provided by the Kasahara Lab. By modeling oncolytic virus therapy in a mouse model that parallels our collection of clinical specimens we have a powerful platform to develop adjuvant therapies and biomarkers of response for oncolytic virus therapy.
Related Publication
Müller S, Kohanbash G, Liu SJ, Alvarado B, Carrera D, Bhaduri A, Watchmaker PB, Yagnik G, Di Lullo E, Malatesta M, Amankulor NM, Kriegstein AR, Lim DA, Aghi M, Okada H, Diaz A. (2017) Single-cell profiling of human gliomas reveals macrophage ontogeny as a basis for regional differences in macrophage activation in the tumor microenvironment. Genome Biology 18(1):234. doi: 10.1186/s13059-017-1362-4.
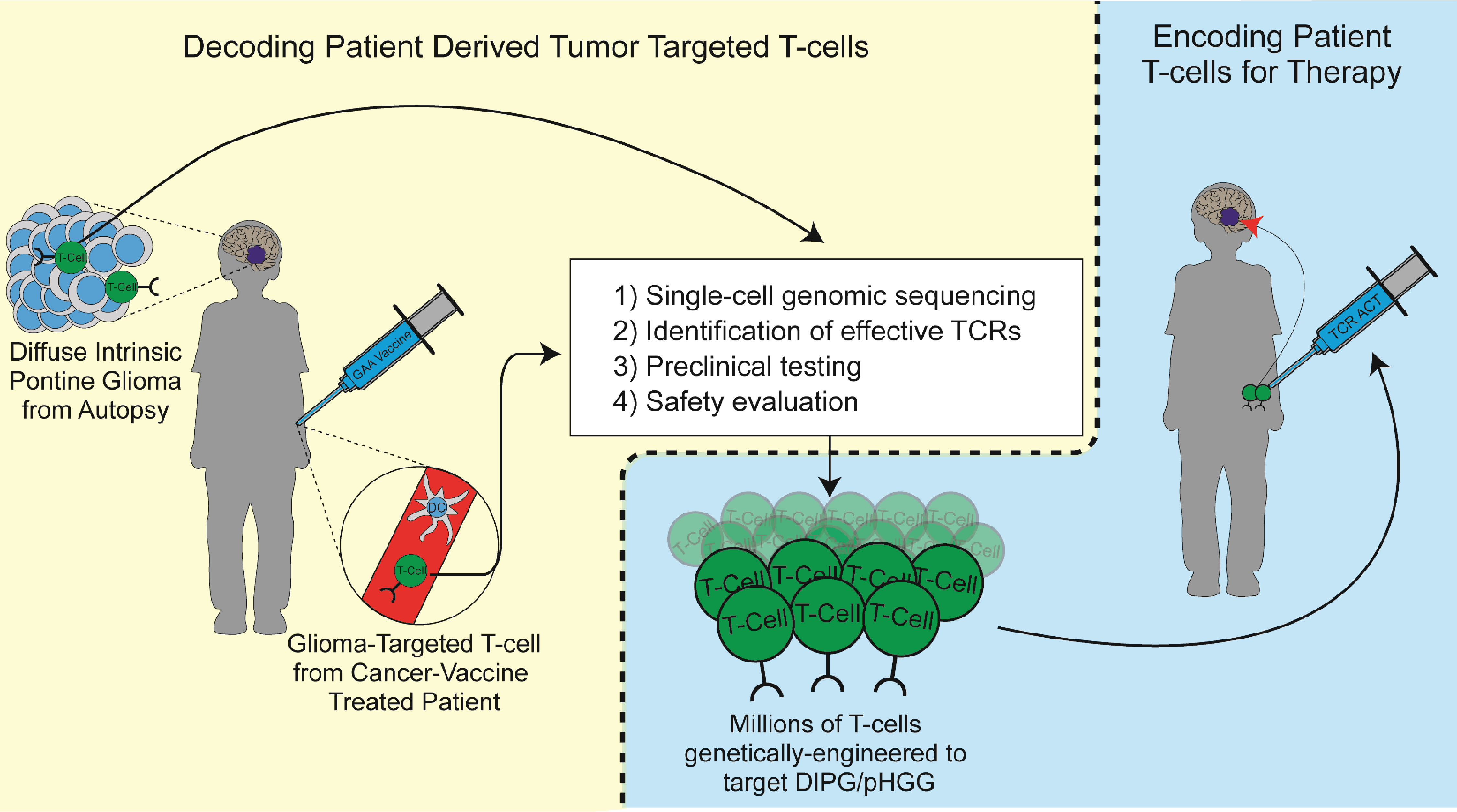
Autologous T-cell Therapy
The Diaz Lab, in collaboration with the Kohanbash Lab at University of Pittsburgh, is investigating T-cell receptor (TCR) therapy for pediatric patients with high-grade glioma (HGG) and diffuse intrinsic pontine glioma (DIPG).
While a patient's immune system may recognize certain tumor-specific molecules (or antigens), the natural immune response is not enough to fully destroy the tumor. With TCR therapy, a patient's own T-cells are extracted from blood or tumor samples and genetically modified to better recognize and attack tumor cells. These modified T-cells are engineered to express TCRs that recognize antigens that are either enriched in tumor cells, or in the case of neoantigens, unique to tumor cells. After a growth period in the lab, millions of modified T-cells are then returned to the patient to help their immune system fight the tumor.
Although TCR therapy has been highly effective in some cancers, finding safe and effective TCRs for pediatric HGG and DIPG has been challenging. We have developed a novel approach for isolating viable TCRs from patient samples. By identifying the few T-cells that successfully target the tumors, we can use single-cell RNA sequencing to determine the most promising TCR sequences.
Isolated TCRs will be further assessed for tumor-specific killing capabilities in both cell culture and animal models. Validating these TCRs is a foundational step towards developing safe and effective TCR therapies for children with HGG and DIPG.
Related Publication
Müller S, Agnihotri S, Shoger KE, Myers MI, Smith N, Chaparala S, Villanueva CR, Chattopadhyay A, Lee AV, Butterfield LH, Diaz A*, Okada H*, Pollack IF*, Kohanbash G*. (2018) Peptide vaccine immunotherapy biomarkers and response patterns in pediatric gliomas. JCI Insight 3(7). pii: 98791. doi: 10.1172/jci.insight.98791. eCollection 2018 Apr 5. *Corresponding authors